Case Studies
Recent Projects
Design Chain of IdentitySolution
working on patient safety
Lonza
Vertex Pharmaceuticals
Location
Houston, USA
Start Date
March 2022
Completed Date
April 2024
About
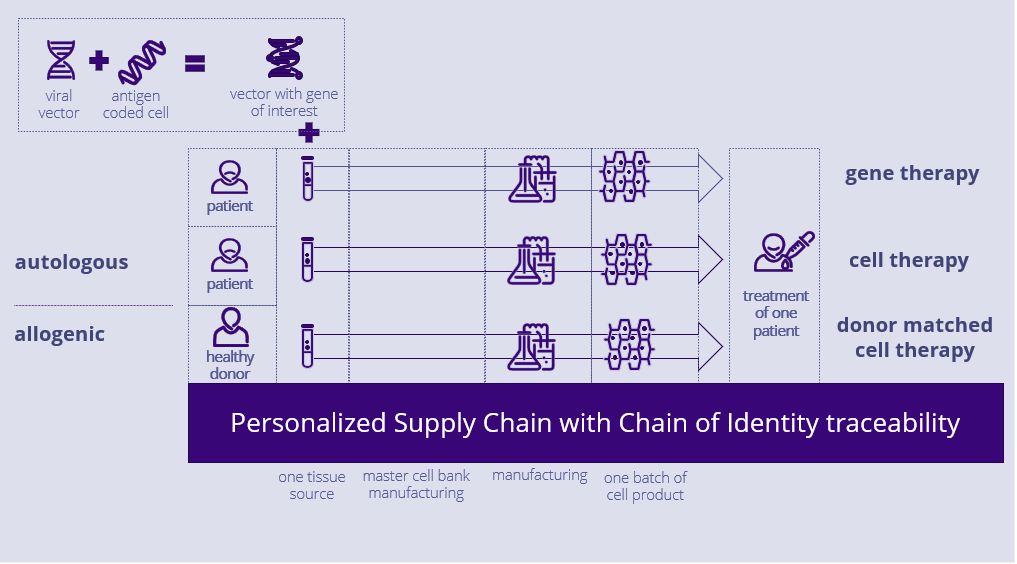
In autologous Cell and Gene Therapies, where each product is patient-specific, the integrity of the Chain of Identity (COI) and Chain of Custody (COC) is critical for ensuring patient safety, quality, and traceability.
From apheresis to infusion, the Chain of Identity guarantees that the correct patient is linked to the correct donor cell and product, providing complete visibility throughout the patient's therapy cycle and across multiple products and doses.
It is essential to accurately assign the right Patient Material batch to the correct process order and sales/delivery order to maintain COI and safeguard patient safety.
The goal of the project was to develop a bi-directional traceability concept that enables to:
- Secure patient safety and comply with regulatory requirements by introducing traceability for the Patient Starting Material batch(es) and the Drug Product batch(es).
- Create a documented system for bi-directional tracking of cells to comply with relevant EU and US regulations on human tissues and cells.
- Material Management (SAP XHA, SAP SC)
- Procurement (SAP S2P)
- Production Planning (SAP P2M)
- Sales & Distribution (SAP O2C)
- Warehouse Management (SAP WH)
- Quality Management (SAP QM)
- Finance and Controlling (SAP R2R)
Challenges

Data Protection and Privacy
To bi-directional trace information, we need to take in account that Sensitive Personal Information needs to be protected within the organization. The challenge was to exclude or replace by non-sensitive identifiers the sensitive personal data in all systems and documentation, like the SAP system, product labels, forms, and batch records.
Sensitive Personal Iinformation encompasses data related to:
- Medical services provided
- A patient's name and address
- The psychological or medical conditions of patients
- A patient's Social Security number and birthdate
This Sensitive Personal Information must be protected as part of healthcare data privacy.


Staggered validation approach
Vertex Pharmaceuticals in the Netherlands and Genentech in the USA were the first clients who supported a seamless bi-directional traceability solution for their autologous cell and gene therapies. To achieve this, a concept for bi-directional traceability in SAP was developed which needed software enhancements.
As bi-directional traceability is a critical GMP process, the validation testing involves the end-to-end processes in multiple scenario's and different validation and go-live stages.
- Test and validate basic software solution in SAP
- Validation of the operational and SAP supported EU processes for Vertex without integration with a Manufacturing Execution System.
- Integration and validation of the SAP solution with a Manufacturing Execution System (MES - MODA) for GenenTech in the USA.
Result
The GMP validated solution enables seamless data exchange and tracking throughout the receiving, manaufacturing and shipping process. This cutting-edge solution enables real-time visibility and control, allowing for accurate tracking of patient-specific products and ensuring compliance with regulatory requirements.

Solutions
Feel free to contact me
