Case Studies
Recent Projects
Electronic Batch Records(EBR)
digitization
Lonza
Vertex Pharmaceuticals
Location
Geleen, The Netherlands
HPortsmouth, USA
Start Date
December 2022
Completed Date
April 2024
About
In the realm of biopharmaceutical manufacturing, Electronic Batch Records (EBR) play a pivotal role in digitizing and streamlining production documentation, ensuring accuracy, compliance, and real-time data integrity. This initiative was structured as a pilot project aimed at delivering a proof of concept for integrating EBR with a Manufacturing Execution System (MES) like MODA, facilitating seamless data flow between enterprise systems and shop-floor operations to reduce manual errors and accelerate batch release.
As a business process architect, the role involved overseeing the secure exchange of data from SAP ERP systems and aligning operational workflows to standard blueprint processes. This ensured robust data security protocols and adherence to best practices for regulatory compliance.
The objectives of the pilot project were to:
- Demonstrate secure, bi-directional data integration between SAP and MODA MES to maintain data integrity and traceability throughout the manufacturing lifecycle as a viable proof of concept.
- Align business processes with standardized blueprints to optimize efficiency, minimize deviations, and support GMP (Good Manufacturing Practices) requirements, validating the approach for potential broader rollout.
Challenges

Data Protection and Privacy
Securing sensitive manufacturing data from SAP while integrating with MODA MES presented significant challenges. This included implementing encryption, access controls, and audit trails to protect proprietary formulations, batch details, and quality metrics. The key was to ensure that data transfers were compliant with regulations like FDA 21 CFR Part 11 and EU Annex 11, without compromising system performance or introducing vulnerabilities.


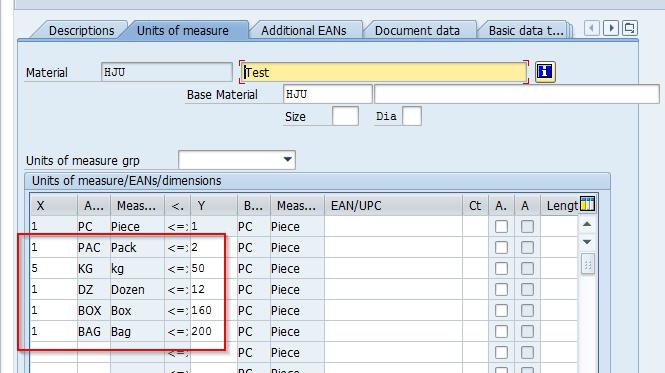
Unit of Measure Alignment
A major challenge in the pilot project was aligning data structures and units of measure between SAP and MODA MES. Discrepancies in how data fields, formats, and measurement units (e.g., volume in liters versus milliliters, or temperature scales) were handled across the systems could lead to errors in batch processing and reporting.
This required detailed mapping exercises, data transformation rules, and validation testing to ensure consistency and accuracy in bi-directional exchanges.

Change control
Determining the leading system for initiating and managing changes was critical to maintain system integrity. SAP was established as the leading system (system of record) for master data changes, such as material specifications or process parameters, due to its role in enterprise-wide planning and compliance.
Change procedures were formalized under GMP guidelines.

Result
The SAP integration with MODA MES, as a successful proof of concept from the pilot project, delivers enhanced manufacturing oversight with secure SAP data flows enabling real-time monitoring, automated reporting, and faster decision-making. This solution ensures full compliance with global regulatory standards, reduces batch review times, and provides a scalable framework for ongoing process improvements and potential full-scale implementation.
The integration has been established as a standard solution blueprint, now ready for adoption by multiple clients to drive operational excellence in biopharma production.

Solutions
Feel free to contact me
